Imaging Core Lab Services
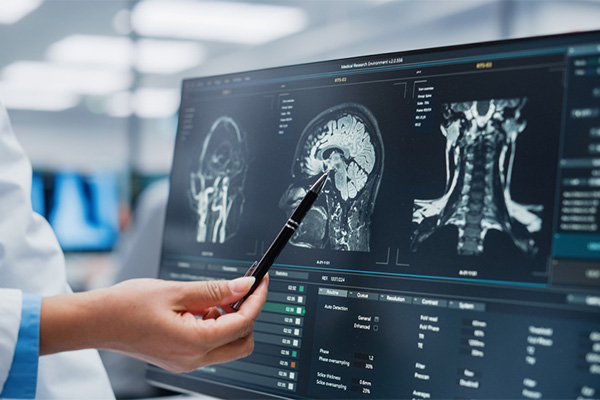
At WCG, we are committed to providing unparallelled imaging core lab services that elevate the quality and efficiency of clinical trials. Utilizing our cutting-edge technology, vast experience, and strong regulatory expertise, we can get studies up and running in as little as one week while ensuring the highest quality imaging data.
Comprehensive Clinical Trial Imaging Services
WCG’s full-service medical imaging core lab provides comprehensive clinical trial imaging services to pharmaceutical, biotechnology, and medical device companies across all therapeutic areas. Navigate the complexities of clinical trials with confidence by partnering with WCG for your imaging needs.
WCG Imaging is an industry leader in conducting multi-case multi-reader studies to evaluate Artificial Intelligence (AI) and Computer Assisted Detection (CAD) systems.
With our team of more than 1,100 board-certified and fellowship-trained physicians, WCG’s Imaging team has sub-specialization in all therapeutic areas, including Hematology/Oncology, Cardiology, CNS, Gastroenterology, Musculoskeletal and Ophthalmology.
Board-certified, fellowship-trained physicians
ISO quality management system certifications
Imaging sites supported in 75 countries
Explore our Imaging Core Lab Solutions
Study Management
Our teams specialize in providing imaging core lab services in support of Phase I-IV Clinical Trials and Class I, II and III Medical Device Trials.
Our Project Managers are experts in study management and have proven records of managing international clinical trials across all therapeutic areas and indications.
We manage all aspects of imaging in clinical trials and ensure to guide it towards success.
Site Management
We specialize in managing international imaging sites participating in Phase I-IV Clinical Trials and Class I, II and III Medical Device Trials.
We currently support over 4,000 imaging sites in 75 countries, and we have extensive experience in qualifying and training imaging sites participating in clinical trials. We manage all clinical trial imaging sites and ensure that all sites are qualified, trained, and able to acquire and submit images as per the image acquisition protocol.
We ensure quality by monitoring all sites throughout the entire trial and quality control each and every timepoint immediately upon receipt to ensure the best possible imaging throughout the entire clinical trial.
Imaging Charter
At WCG Imaging, our team of more than 1,100 board-certified and fellowship-trained physicians, are experts in all therapeutic areas.
Our team has expertise in Oncology, Cardiology, Central Nervous System, Gastroenterology, Musculoskeletal and Ophthalmology and their indications. This expertise allows us to develop a sophisticated and optimized Imaging Charter with the appropriate review criteria.
At WCG Imaging, we ensure all of our reviewers are experienced, appropriately trained and have the expertise needed to ensure quality assessments of even the most complex of indications.
Image Acquisition Protocol
We recognize it is essential to ensure the correct acquisition of each participant’s imaging timepoint.
Our ISO certified processes ensure that the processing of participant images received from sites is meticulously planned, implemented and monitored for success.
Whether the images received are in support of a drug, medical device, or artificial intelligence (AI) clinical trial, our team has the expertise and experience to ensure quality and compliance of every participant scan.
Case Review
Our team consists of more than 1,100 full time and consulting board-certified, fellowship-trained physicians and key opinion leaders (KOLs) with sub-specialization in all therapeutic areas.
Using our ISO certified process, we plan, implement and monitor case reviews meticulously to ensure success. All of our reviewers are experienced, appropriately trained and have the expertise needed to ensure quality assessments of even the most complex of indications.
Whether the review is in support of a Phase I-IV clinical trial or in support of a Class I, II or III trial to study a medical device, our physicians have the expertise and experience to provide comprehensive services to pharmaceutical, biotechnology and medical device companies worldwide.
Data Management
Our technology allows us to provide robust data management and accommodate even the most specialized clinical and medical device trials.
Our data management team ensures that the case report, database and data exports are designed and configured optimally to ensure the data is acquired, validated, stored, protected, and processed as expected to ensure the accessibility, reliability, and timeliness of the data.
In addition to having one of the most comprehensive and sophisticated quality management systems, and leading the industry with 3 ISO certifications, our data management team also complies Good Automated Manufacturing Practice (GAMP®5).
Who We Serve
Pharmaceutical/Biotechnology
At WCG Imaging, our team consists of more than 1,100 board-certified, fellowship-trained physicians and key opinion leaders (KOLs) with sub-specialization in all therapeutic areas.
Our therapeutic expertise includes, but is not limited to Oncology, Cardiology, Central Nervous System, Gastroenterology, Musculoskeletal and Ophthalmology.

ISO Certified Quality Management System
ISO Certified Imaging Core Lab
WCG Imaging has one of the most comprehensive and sophisticated quality management systems in the industry:
- Only imaging core lab in the world to hold three ISO quality management system certifications specifically for providing imaging core lab services on clinical trials.
- Inspected by the FDA with Zero 483s.
- Our quality management system is certified by the British Standards Institute (BSI).
Quality Management System
Unique in the industry, our quality management system is certified to ISO 9001, ISO 13485, and ISO 27001 by the British Standards Institute (BSI). Our certifications are specifically for providing imaging core lab services on clinical trials.
In addition to our team, technology and therapeutic expertise, our ISO-certified quality management system also provides Sponsors with high confidence in our abilities as a leading medical imaging core lab.

ISO 9001
Quality Management
IS0 13485
Medical Device Quality Management
ISO 27001
Information Security Management
Explore our therapeutic expertise:
Contact WCG Imaging to discuss your trial’s imaging needs
We have the team, therapeutic expertise, technology, and ISO-certified quality management systems to provide imaging core lab services to our clients worldwide. Complete the form to get started.